Question
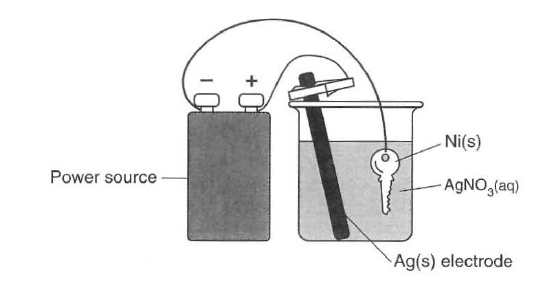
The diagram below represents an operating electrolytic cell used to plate silver onto a nickel key. As the cell operates, oxidation occurs at the silver electrode and the mass of the silver electrode decreases.
60 Identify the cathode in the cell. [1]HIGHLIGHT TO SEE THE ANSWER
61 State the purpose of the power source in the cell. [1]HIGHLIGHT TO SEE THE ANSWER
62 Explain, in terms of Ag atoms and Ag+(aq) ions, why the mass of the silver electrode decreases as the cell operates. [1]HIGHLIGHT TO SEE THE ANSWER
|
on to Questions 63-65