Question
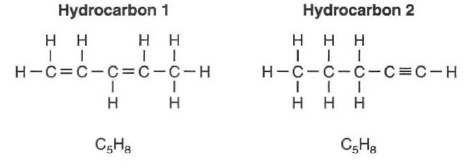
Two hydrocarbons that are isomers of each other are represented by the structural formulas and molecular formulas below.
58 Explain, in terms of bonds, why these hydrocarbons are unsaturated. [1]HIGHLIGHT TO SEE THE ANSWER
59 Explain, in terms of structural formulas and molecular formulas, why these hydrocarbons are isomers of each other. [1]HIGHLIGHT TO SEE THE ANSWER
|
on to Questions 60-62