Questions | Answer | Explanations |
11 Which change occurs when a barium atom loses two electrons?
| 3 | losing electrons is oxidation (becomes more +) Radius decreases |
12 Conductivity in a metal results from the metal atoms having
| 4 | Electricity Conducting "Moving Charge" metals=electrons moving electrolytes= ions moving |
| 13 Which of these elements has the least attraction for electrons in a chemical bond?
| 3 | Definition of Electronegativity |
| 14 Recovering the salt from a mixture of salt and water could best be accomplished by
| 1 | boil away the water leaving salt |
15 The average kinetic energy of water molecules is greatest in which of these samples?
| 2 | highest temperature (avg KE) |
16 Helium is most likely to behave as an ideal gas when it is under
| 3 | ideal gases occur at low pressures and high temperatures same conditions as an ideal vacation |
17 At STP, the element oxygen can exist as either O2 or O3 gas molecules. These two forms of the element have
| 4 | allotropes, same element different form different chemical and physical properties diamond graphite |
18 Which sample contains particles in a rigid, fixed, geometric pattern?
| 4 | solid-fixed geometric pattern |
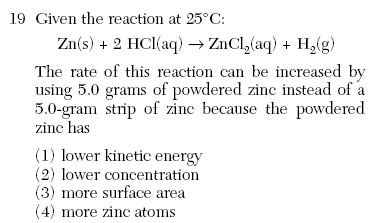 | 3 | powder increases surface area |
| 20 Which statement about a system at equilibrium is true? (1) The forward reaction rate is less than the reverse reaction rate. | 3 | equilibrium concentrations are constant or rates are equal |