Questions | Answer | Explanations |
21 A catalyst increases the rate of a chemical reaction by
| 1 | definition of a catalyst lowers activation energy |
22 Which element must be present in an organic compound?
| 3 | carbon |
23 Which compound is a saturated hydrocarbon?\
| 1 | saturated all single bonds alkanes CnH2n+2 |
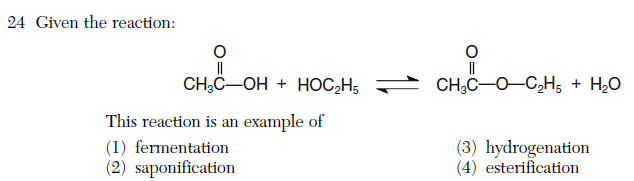 | 4 | an ester is produced from an alcohol and acid --> Esterfication |
25 Which of these compounds has chemical properties most similar to the chemical properties of ethanoic acid?
| 1 | the other acid |
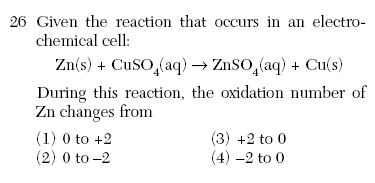 | 1 | Zn0 to Zn+2 (SO42-) |
27 A voltaic cell spontaneously converts
| 2 | Voltaic Spontaneous (A Battery) Electrolytic Nonspontaneous (Apply a current) |
28 Which pair of formulas represents two compounds that are electrolytes?
| 2 | Electrolytes Acids, bases and Salts **Never Alcohols CH3OH |
29 Hydrogen chloride, HCl, is classified as an Arrhenius acid because it produces
| 1 | Acid H+ Base OH- |
30 Which compound could serve as a reactant in a neutralization reaction?
| 2 | Acid or Base |