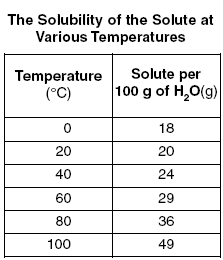
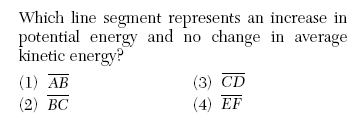Questions | Answer | Explanations |
31 Which of these particles has the greatest mass?
| 1 | Alpha then neutron then beta/positron |
32 In a nuclear fusion reaction, the mass of the products is
| 1 | in nuclear reactions mass converts to energy E=mc^2 |
33 Which of these types of radiation has the greatest penetrating power?
| 3 | gamma> positron/beta>alpha |
34 How many electrons are contained in an Au3+ ion?
| 1 | positive ions lose electrons 79-3=76 |
35 Which electron configuration represents the electrons of an atom in an excited state?
| 3 | Excited state promotes an electron to a higher energy level 2-7-2 should be 2-8-1 |
36 In comparison to an atom of F-19 in the ground state, an atom of C-12 in the ground state has
| 2 | F-19 has 10 n, 9 e-, 9p+ C-12 has 6 n, 6 e-, 6 p+ 3 less valence electrons
|
37 Element X is a solid that is brittle, lacks luster, and has six valence electrons. In which group on the Periodic Table would element X be found?
| 4 | 6 valence electrons group 16 |
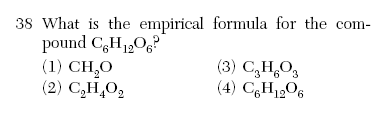 | 1 | empirical formula is the lowest ratio of atoms |
39 The bonds between hydrogen and oxygen in a water molecule are classified as
| 1 | small difference in electronegativities between H and O |
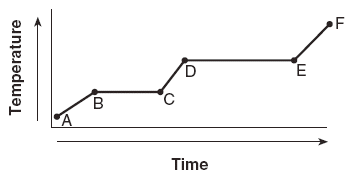
| 40 The graph below represents the uniform heating of a substance, starting with the substance as a solid below its melting point.
| 2 | average KE= temperature no change is BC or DE |