Questions | Answer | Explanations |
41 Using your knowledge of chemistry and the information in Reference Table H, which statement concerning propanone and water at 50°C is true?
| 2 | more vapor weaker intermolecular forces |
42 A solution that is at equilibrium must be
| 3 | a solution is saturated when it is at equilibrium dissolving and crystallizing are occurring at the same time |
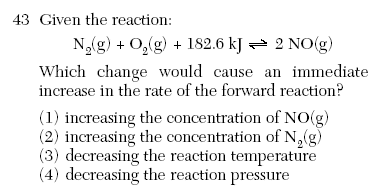 | 2 | increasing surface area, concentration, temperature or catalyst increase reaction rate this is not LeChaterlier because it is not at equilibrium |
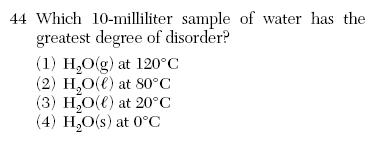 | 1 | Entropy gases>liquids>solids |
45 Which pH indicates a basic solution?
| 4 | Bases above 7 acids below 7 |
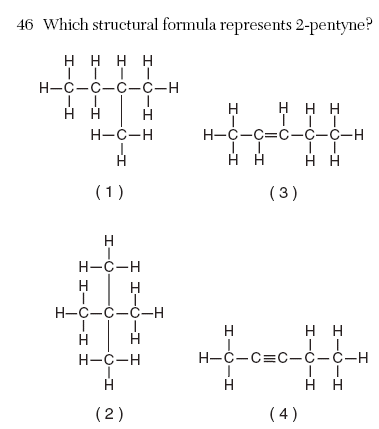 | 4 | 5 carbons the triple bond is on 2 |
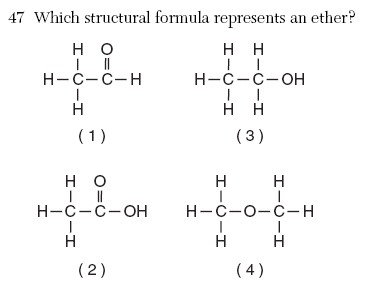 | 4 | use Reference tables |
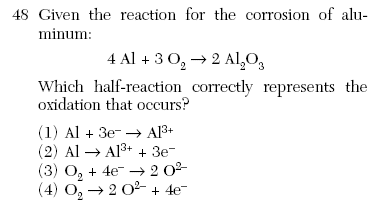 | 2 | oxidation charge goes up electrons on the more positive side |
 | 2 | 2.69d is the half live so it will be reduced by 1/2 |
50 As the elements of Group 1 on the Periodic Table are considered in order of increasing atomic radius, the ionization energy of each successive element generally
| 1 | going down group 1 it is easier to remove electrons as they are further from the nucleus |