Questions | Answer | Explanations |
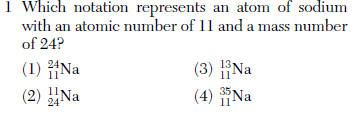 | 1 | Use table N for help |
| 2 Which element has chemical properties that are most similar to those of calcium? (1) Co (3) N (2) K (4) Sr | 4 | same group |
| 3 Which element is malleable and can conduct electricity in the solid phase? (1) iodine (3) sulfur (2) phosphorus (4) tin | 4 | the metal |
| 4 Atoms of different isotopes of the same element differ in their total number of (1) electrons (3) protons (2) neutrons (4) valence electrons | 2 | definition of Isotope |
| 5 Which statement correctly describes two forms of oxygen, O2 and O3? (1) They have identical molecular structures and identical properties. (2) They have identical molecular structures and different properties. (3) They have different molecular structures and identical properties. (4) They have different molecular structures and different properties. | 4 | different properties based on different structures |
| 6 What is the IUPAC name for the compound FeS? (1) iron(II) sulfate (3) iron(II) sulfide (2) iron(III) sulfate (4) iron(III) sulfide | 3 | Fe2+ S2- iron(II) sulfide |
| 7 Given the balanced equation representing a reaction: F2(g) + H2(g) ==> 2HF(g) What is the mole ratio of H2(g) to HF(g) in this reaction? (1) 1:1 (3) 2:1 (2) 1:2 (4) 2:3 | 2 | use the coefficients (the numbers in front) |
| 8 Which list includes three types of chemical reactions? (1) condensation, double replacement, and sublimation (2) condensation, solidification, and synthesis (3) decomposition, double replacement, and synthesis (4) decomposition, solidification, and sublimation | 3 | chemical are NOT phase changes |
| 9 Which type of bond results when one or more valence electrons are transferred from one atom to another? (1) a hydrogen bond (2) an ionic bond (3) a nonpolar covalent bond (4) a polar covalent bond | 2 | transferred-- ionic |
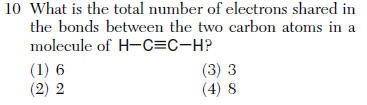 | 1 | 6 electrons shared or 3 pairs (not the question) |
Questions 1-10 Questions 11-20 Questions 21-30 Questions 31-40 Questions 41-50