Questions | Answer | Explanations |
41 Which Kelvin temperature is equivalent to –24°C?
(1) 226 K (3) 273 K
(2) 249 K (4) 297 K | 2 | K=C + 273 Table T |
42 Which substance has the lowest vapor pressure at 75°C?
(1) water (3) propanone
(2) ethanoic acid (4) ethanol | 2 | Table H |
43 What is the IUPAC name for the compound that has the condensed structural formula CH3CH2CH2CHO?
(1) butanal (3) propanal
(2) butanol (4) propanol | 1 | draw it out 4 carbons but- -al CH=O |
44 What volume of 0.500 M HNO3(aq) must completely react to neutralize 100.0 milliliters of 0.100 M KOH(aq)?
(1) 10.0 mL (3) 50.0 mL
(2) 20.0 mL (4) 500. mL | 2 | titration MV=MV Table T |
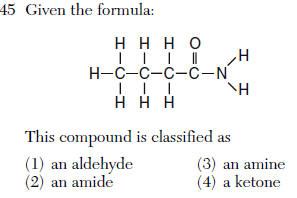 | 2 | Table R |
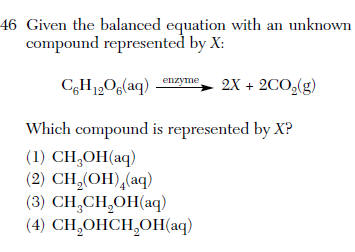 | 3 | Fermentation Ethanol is produced or find the missing atoms |
47 Which reactants form the salt CaSO4(s) in a neutralization reaction?
(1) H2S(g) and Ca(ClO4)2(s)
(2) H2SO3(aq) and Ca(NO3)2(aq)
(3) H2SO4(aq) and Ca(OH)2(aq)
(4) SO2(g) and CaO(s) | 3 | H2SO4(aq)+ Ca(OH)2(aq)==> CaSO4(s) +H2O(l) |
48 A student tested a 0.1 M aqueous solution and made the following observations:
• conducts electricity
• turns blue litmus to red
• reacts with Zn(s) to produce gas bubbles
Which compound could be the solute in this solution?
(1) CH3OH (3) HBr
(2) LiBr (4) LiOH | 3 | Must be an Acid |
49 What is the half-life of sodium-25 if 1.00 gram of a 16.00-gram sample of sodium-25 remains unchanged after 237 seconds?
(1) 47.4 s (3) 79.0 s
(2) 59.3 s (4) 118 s | 2 | 16-->8-->4-->2-->1 4 half lives 237s/4= |
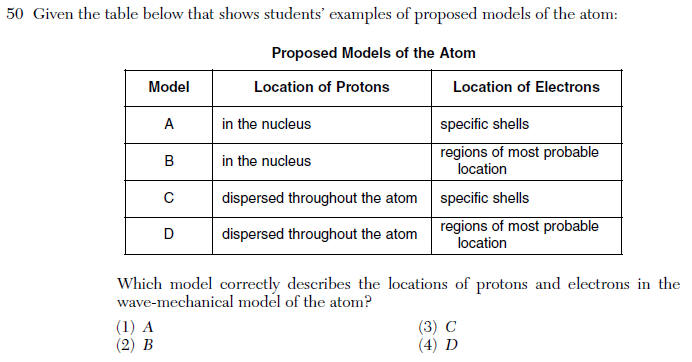 | 2 | wave model=electron locations are base on probability |


