Questions | Answer | Explanations |
31 Which trends are observed as each of the elements within Group 15 on the Periodic Table is considered in order from top to bottom?
(1) Their metallic properties decrease and their atomic radii decrease.
(2) Their metallic properties decrease and their atomic radii increase.
(3) Their metallic properties increase and their atomic radii decrease.
(4) Their metallic properties increase and their atomic radii increase. | 4 | Group 15 top nonmetal bottom metal also use Table S from radii |
32 What is the total number of electrons in a S2– ion?
(1) 10 (3) 16
(2) 14 (4) 18 | 4 | Sulfur atom has 16 electrons S2– ion has 18 electrons |
33 A substance has an empirical formula of CH2 and a molar mass of 56 grams per mole. The molecular formula for this compound is
(1) CH2 (3) C4H8
(2) C4H6 (4) C8H4 | 3 | C4H8 =56g/mol |
34 Compared to an atom of phosphorus-31, an atom of sulfur-32 contains
(1) one less neutron (3) one more neutron
(2) one less proton (4) one more proton | 4 | phosphorus-31 P=15 n=16 sulfur-32 P= 16 n=16 |
35 In which compound is the percent compositionby mass of chlorine equal to 42%?
(1) HClO (gram-formula mass = 52 g/mol)
(2) HClO2 (gram-formula mass = 68 g/mol)
(3) HClO3 (gram-formula mass = 84 g/mol)
(4) HClO4 (gram-formula mass = 100. g/mol) | 3 | (35.5/84) x 100%=42% |
36 A metal, M, forms an oxide compound with the general formula M2O. In which group on the Periodic Table could metal M be found?
(1) Group 1 (3) Group 16
(2) Group 2 (4) Group 17 | 1 | M= +1 group 1 |
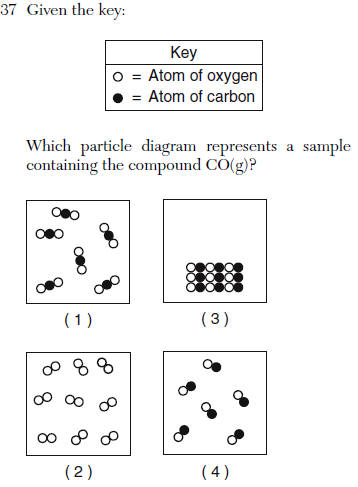 | 4 | 2 different atoms bonded, spread out (gas) |
38 At standard pressure, which element has a melting point higher than standard temperature?
(1) F2 (3) Fe
(2) Br2 (4) Hg | 3 | Table S |
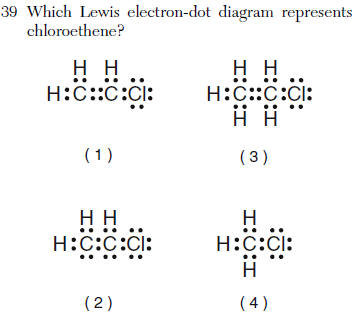 | 1 | chloro -a chlorine atom eth- 2 carbons -ene double bond (2 pairs of shared electrons) |
40 A saturated solution of NaNO3 is prepared at 60.°C using 100. grams of water. As this solution is cooled to 10.°C, NaNO3 precipitates (settles)
out of the solution. The resulting solution is saturated. Approximately how many grams of NaNO3 settled out of the original solution?
(1) 46 g (3) 85 g
(2) 61 g (4) 126 g | 1 | At 60 125g dissolved- at 10 75g dissolved= 45g precipitates |

