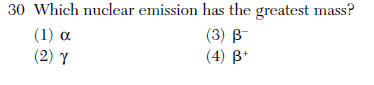Questions
(1) the activation energies of the forward and reverse reactions
(2) the rates of the forward and reverse reactions
(3) the concentrations of the reactants and products
(4) the potential energies of the reactants and products
concentration constant
rates are equal
(1) density (3) melting point
(2) reactivity (4) molecular formula
(1) AgNO3 + NaCl ==>AgCl + NaNO3
(2) BaCl2 + K2CO3 Å==> BaCO3 + 2KCl
(3) CuO + CO==> Cu + CO2
(4) HCl + KOH Å==> KCl + H2O
(1) the loss of protons
(2) the loss of electrons
(3) the gain of protons
(4) the gain of electrons
Red Cat, GER gain electrons
(1) CH3OH (3) H2O
(2) C6H12O6 (4) KOH
KOH is a base
(1) hydride ion (3) hydronium ion
(2) hydrogen ion (4) hydroxide ion
(1) C-12 and N-14 (3) C-14 and N-14
(2) C-12 and N-16 (4) C-14 and N-16
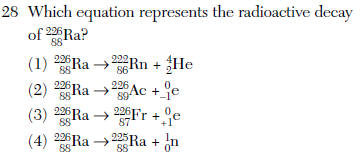
it is an alpha decay
(1) neutralization (3) substitution
(2) polymerization (4) transmutation