Questions
(1) 1 electron (3) 3 electrons
(2) 2 electrons (4) 4 electrons
(1) temperature (3) thermal energy
(2) pressure (4) chemical energy
(1) 3.0 grams of HCl per liter of water
(2) 3.0 grams of HCl per mole of solution
(3) 3.0 moles of HCl per liter of solution
(4) 3.0 moles of HCl per mole of water
M=Molarity=mol/L
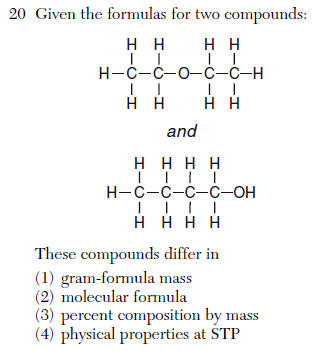
(1) homogeneous compound
(2) homogeneous mixture
(3) heterogeneous compound
(4) heterogeneous mixture
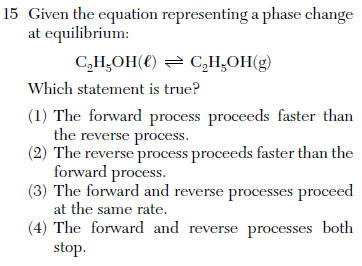
concentrations are constant and rates are equal
(1) a zinc strip and 1.0 M HCl(aq)
(2) a zinc strip and 3.0 M HCl(aq)
(3) zinc powder and 1.0 M HCl(aq)
(4) zinc powder and 3.0 M HCl(aq)
high concentration 3.0M vs 1.0M
(1) providing an alternate reaction pathway that has a higher activation energy
(2) providing an alternate reaction pathway that has a lower activation energy
(3) using the same reaction pathway and increasing the activation energy
(4) using the same reaction pathway and decreasing the activation energy
(1) 2CO(g) + O2(g)==> 2CO2(g)
(2) 4Al(s) + 3O2(g) ==>2Al2O3(s)
(3) 2H2(g) + O2(g)==> 2H2O(g)
(4) N2(g) + 3H2(g) ==>2NH3(g)
(1) 1 (3) 3
(2) 2 (4) 4