Questions
(1) stronger covalent bonds
(2) stronger intermolecular forces
(3) weaker covalent bonds
(4) weaker intermolecular forces
(1) 25°C (3) 35°C
(2) 30.°C (4) 40.°C
from a solid to a liquid?
(1) 874°C (3) 328°C
(2) 601°C (4) 0°C
N2(g) + 3H2(g)==> 2NH3(g) + energy
Which change causes the equilibrium to shift to the right?
(1) decreasing the concentration of H2(g)
(2) decreasing the pressure
(3) increasing the concentration of N2(g)
(4) increasing the temperature
Pressure forces a shift to the side with the lower moles of gases
(1) hexanal (3) hexanoic acid
(2) hexane (4) hexyne
hydrocarbon hydrogen and carbon only
Table Q
is classified as an
(1) alcohol (3) ester
(2) aldehyde (4) ether
2Al3+(aq) + 3Mg(s)==>3Mg2+(aq) + 2Al(s)
In this reaction, electrons are transferred from
(1) Al to Mg2+ (3) Mg to Al3+
(2) Al3+ to Mg (4) Mg2+ to Al
ox. to red
NOT VOLTAIC CELL QUESTION
(1) CH3COOH and CH3CH2OH
(2) HC2H3O2 and H3PO4
(3) KHCO3 and KHSO4
(4) NaSCN and Na2S2O3
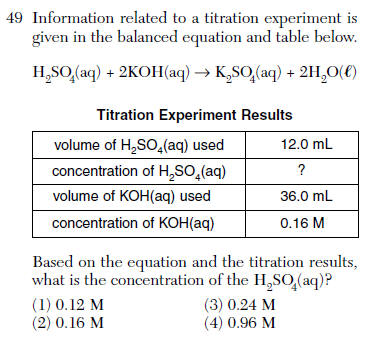
it is MV=2MV
(36mL)(0.16M)=2M(12.0mL)
(1) cobalt-60 (3) phosphorus-32
(2) iodine-131 (4) uranium-238