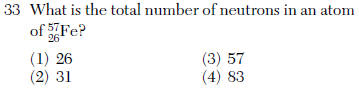Questions | Answer | Explanations |
31 Which electron configuration could represent a strontium atom in an excited state?
(1) 2𤾃8𤪗 (3) 2𤾃8𤾃
(2) 2𤾃8𤪙 (4) 2𤾃8𤾄 | 2 | 38 electrons NOT 2-8-18-8-2 |
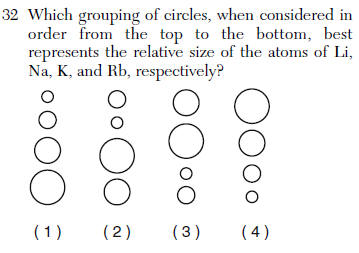 | 1 | Use Table S or know the falling down snowman trick |
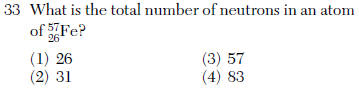 | 2 | 57-26= neutrons |
34 At STP, which element is brittle and not a conductor of electricity?
(1) S (3) Na
(2) K (4) Ar | 1 | nonmetal properties |
35 What is the total number of electrons in a
Mg2+ ion? (1) 10 (3) 14
(2) 12 (4) 24 | 1 | Mg atom 12 electrons Mg2+ ion has lost 2 so it has 10 electrons |
36 Which formula represents lead(II) chromate?
(1) PbCrO4 (3) Pb2CrO4
(2) Pb(CrO4)2 (4) Pb2(CrO4)3 | 1 | Pb2+ CrO42- 1:1 ratio |
37 Compared to an electron in the first electron shell of an atom, an electron in the third shell of the same atom has
(1) less mass (3) more mass
(2) less energy (4) more energy | 4 | higher energy level more energy...duh |
38 Which pair consists of a molecular formula and its corresponding empirical formula?
(1) C2H2 and CH3CH3 (3) P4O10 and P2O5
(2) C6H6 and C2H2 (4) SO2 and SO3 | 3 | empirical is a reduced form of the molecular |
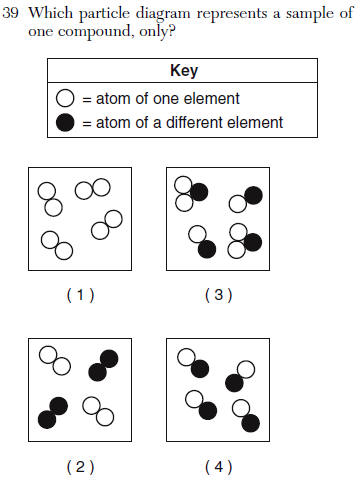 | 4 | Must be 2 different elements attached all compounds must be the same |
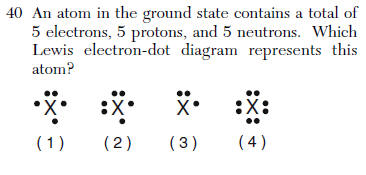 | 3 | Mean Question 5 protons is Boron electron configuration is 2-3 3 dots |