Questions
(1) pentane (3) pentyne
(2) pentene (4) pentanol
-ene
(1) The Pt2+ gains electrons and its oxidation number increases.
(2) The Pt2+ gains electrons and its oxidation number decreases.
(3) The Pt2+ loses electrons and its oxidation number increases.
(4) The Pt2+ loses electrons and its oxidation number decreases.
gain electrons reduction
oxidation number decrease
(1) BaCl2 + Na2SO4==>BaSO4 + 2NaCl
(2) C+H2O ==>CO + H2
(3) CaCO3==>CaO + CO2
(4) Mg(OH)2 + 2HNO3==>Mg(NO3)2 + 2H2O
(1) Chemical energy is spontaneously converted to electrical energy.
(2) Chemical energy is converted to electrical energy only when an external power source is provided.
(3) Electrical energy is spontaneously converted to chemical energy.
(4) Electrical energy is converted to chemical energy only when an external power source is provided.
(1) hydride ion (3) hydronium ion
(2) hydrogen ion (4) hydroxide ion
(1) accepts an H+ (3) donates an H+
(2) accepts an OH– (4) donates an OH–
(1) C-14, N-16, P-32
(2) Cs-137, Fr-220, Tc-99
(3) Kr-85, Ne-19, Rn-222
(4) Pu-239, Th-232, U-238
(1) alpha particle (3) gamma radiation
(2) beta particle (4) positron
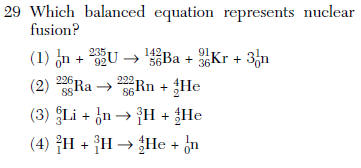
REGENTS ONLY use hydrogen
(1) breaking of bonds between atoms
(2) formation of bonds between atoms
(3) conversion of mass into energy
(4) conversion of energy into mass