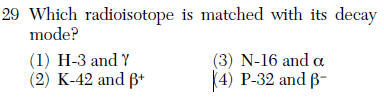Questions | Answer | Explanations |
21 A molecule of an organic compound contains at least one atom of
(1) carbon (3) nitrogen
(2) chlorine (4) oxygen | 1 | organic = carbon |
22 In a chemical reaction, the difference between the potential energy of the products and the potential energy of the reactants is equal to the
(1) activation energy
(2) entropy of the system
(3) heat of fusion
(4) heat of reaction | 4 | definition of heat of reaction or delta H |
23 A carbon-carbon triple bond is found in a molecule of
(1) butane (3) butene
(2) butanone (4) butyne | 4 | -yne contain triple bonds (Table Q) |
24 Which statement describes one characteristic of an operating electrolytic cell?
(1) It produces electrical energy.
(2) It requires an external energy source.
(3) It uses radioactive nuclides.
(4) It undergoes a spontaneous redox reaction. | 2 | electrolytic is Nonspontaneous and requires electricity |
25 Which compound when dissolved in water is an Arrhenius acid?
(1) CH3OH (3) NaCl
(2) HCl (4) NaOH | 2 | Arrhenius acids start with H |
26 An acid can be defined as an
(1) H+ acceptor (3) OH- acceptor
(2) H+ donor (4) OH- donor | 2 | Acids donate H+ Bases accept it |
27 Which nuclear emission has no charge and no mass?
(1) alpha particle (3) gamma ray
(2) beta particle (4) positron | 3 | gamma Table O |
28 During which process can 10.0 milliliters of a 0.05 M HCl(aq) solution be used to determine the unknown concentration of a given volume of NaOH(aq) solution?
(1) evaporation (3) filtration
(2) distillation (4) titration | 4 | Definition of a titration |
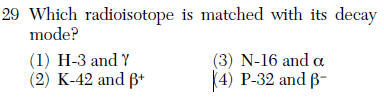 | 4 | Use Table N |
30 Which reaction is accompanied by the release of the greatest amount of energy?
(1) combustion of 10. g of propane
(2) electrolysis of 10. g of water
(3) nuclear fission of 10. g of uranium
(4) oxidation of 10. g of iron | 3 | Always Nuclear E=mc2 |