Questions
(1) 400. K to 200. K (3) 400.°C to 200.°C
(2) 200. K to 400. K (4) 200.°C to 400.°C
(1) barium phosphate (3) silver iodide
(2) calcium sulfate (4) sodium perchlorate
N2(g) + 3H2(g)<==>2NH3(g) + energy
Which changes occur when the temperature of this system is decreased?
(1) The concentration of H2(g) increases and the concentration of N2(g) increases.
(2) The concentration of H2(g) decreases and the concentration of N2(g) increases.
(3) The concentration of H2(g) decreases and the concentration of NH3(g) decreases.
(4) The concentration of H2(g) decreases and the concentration of NH3(g) increases.
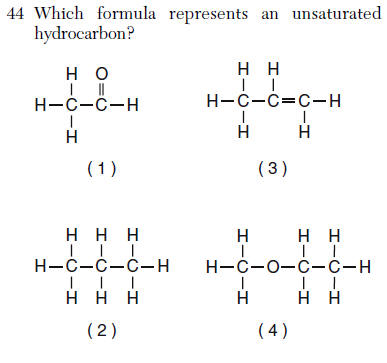
hydrocarbon= hydrogen and carbon ONLY
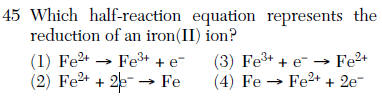
(1) Ag (3) Cu
(2) Au (4) Pb
Zn(s) + Cu2+(aq)==> Zn2+(aq) + Cu(s)
The flow of electrons through the external circuit in this cell is from the
(1) Cu anode to the Zn cathode
(2) Cu cathode to the Zn anode
(3) Zn anode to the Cu cathode
(4) Zn cathode to the Cu anode
Oxidation (anode) to reduction (cathode)
Charge going to charge going down
(1) pH of KCl(aq)
(2) pH of KCl(s)
(3) electrical conductivity of KCl(aq)
(4) electrical conductivity of KCl(s)
(1) CaCl2 (3) HClO
(2) CaH2 (4) HClO2
Hint Ca + Cl
(1) C-14 and C-12 (3) I-131 and Xe-131
(2) Co-60 and Co-59 (4) U-238 and Pb-206