Questions | Answer | Explanations |
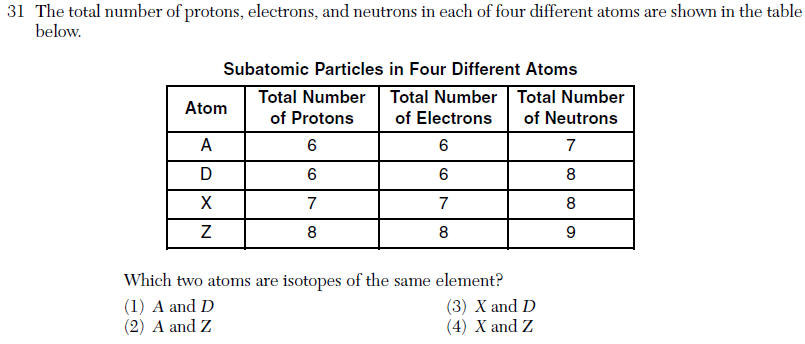 | 1 | Isotopes same number of protons different number of neutrons |
 | 3 | 3 valence electrons |
33 Which element forms a compound with chlorine with the general formula MCl?
(1) Rb (3) Re
(2) Ra (4) Rn | 1 | Cl is 1- so M must be 1+ so it is in group 1 |
34 A sample of an element has a mass of 34.261 grams and a volume of 3.8 cubic centimeters. To which number of significant figures should the calculated density of the sample be expressed?
(1) 5 (3) 3
(2) 2 (4) 4 | 2 | dividing (the sig figs will match the least number of sig figs used 3.8 has only 2 sig figs |
35 Which characteristics both generally decrease when the elements in Period 3 on the Periodic Table are considered in order from left to right?
(1) nonmetallic properties and atomic radius
(2) nonmetallic properties and ionization energy
(3) metallic properties and atomic radius
(4) metallic properties and ionization energy | 3 | left side are metals right are nonmetals use table S for radius |
36 Which formula is both a molecular and an empirical formula?
(1) C6H12O6 (3) C3H8O
(2) C2H4O2 (4) C4H8 | 3 | Empirical is fully reduced to the simplest whole number ratio |
37 An atom of argon in the ground state tends not to bond with an atom of a different element because the argon atom has
(1) more protons than neutrons
(2) more neutrons than protons
(3) a total of two valence electrons
(4) a total of eight valence electrons | 4 | full valence shell of 8 electrons is stable and generally unreactive |
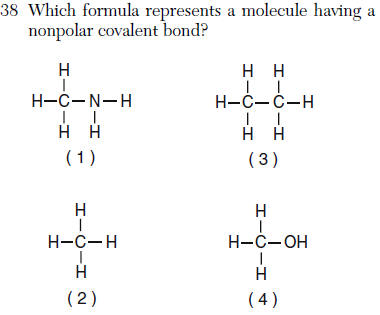 | 3 | HARDEST Question the C-C bond is nonpolar |
39 Which compound has the lowest vapor pressure at 50°C?
(1) ethanoic acid (3) propanone
(2) ethanol (4) water | 1 | Table H |
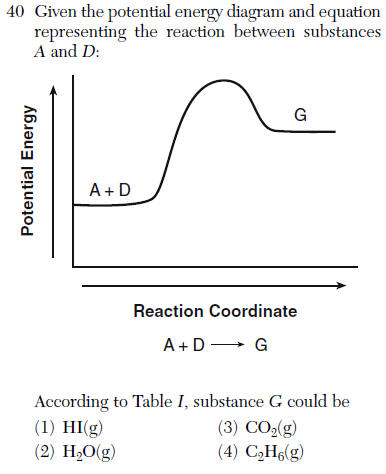 | 1 | Find the endothermic reaction Delta H will be positive, the product of this is the answer |



