Questions
Explanations
31 Which notations represent hydrogen isotopes?

Different number of neutrons (mass number is protons and neutrons... top)
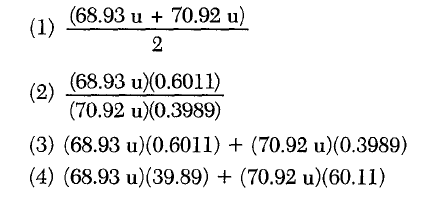
(1) B, Al, Ga (3) C, Si, Ge
(2) Li, Be, B (4) P, S, Cl
34 In the formula XSO4, the symbol X could represent the element
(1) Al (3) Mg
(2) Ar (4) Na
look at group 2...Mg
(1) PbO2 (2) Pb2O
(3) PbO4 (4) Pb4O
Pb4+ O2-
PbO2