Questions
Explanations
(1) Both electronegativity and atomic radius increase.
(2) Both electronegativity and atomic radius decrease.
(3) Electronegativity increases and atomic radius decreases.
(4) Electronegativity decreases and atomic radius increases.
37 What is the percent composition by mass of nitrogen in (NH4)2CO3 (gram-formula mass =96.0 g/mol)?
(1) 14.6% (2) 29.2% (3) 58.4% (4) 87.5%
2KI + F2 ==> 2KF + I2
What type of chemical reaction does this equation represent?
(1) synthesis
(2) decomposition
(3) single replacement
(4) double replacement
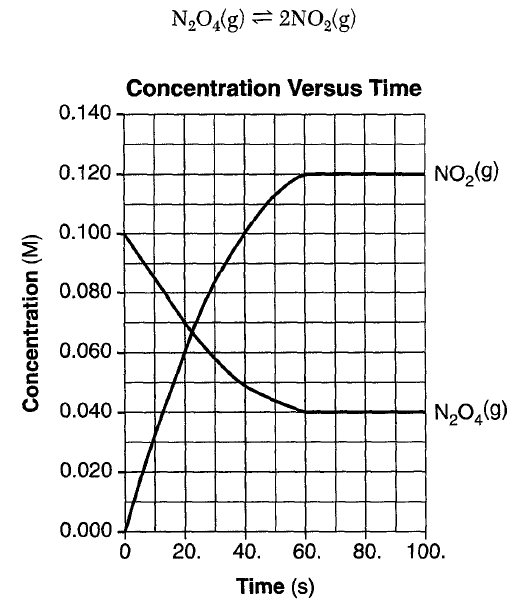
The graph shows that the reaction is at equilibrium after 60. seconds because the concentrations of both NO2(g) and N2O4(g) are
(1) increasing (3) constant
(2) decreasing (4) zero
Concentrations are constant
or Rates are equal
@ equailibirium