Questions | Answer | Explanations |
1 What is the total number of valence electrons in a calcium atom in the ground state?
(1) 8 (3) 18
(2) 2 (4) 20 | 2 | 2-8-8-2 the last number |
2 Which subatomic particles are located in the nucleus of an He-4 atom?
(1) electrons and neutrons
(2) electrons and protons
(3) neutrons and protons
(4) neutrons, protons, and electrons | 3 | nucleus contains protons and neutrons |
3 In the late 1800s, experiments using cathode ray tubes led to the discovery of the
(1) electron (3) positron
(2) neutron (4) proton | 1 | cathode rays are electrons |
4 The atomic mass of titanium is 47.88 atomic mass units. This atomic mass represents the
(1) total mass of all the protons and neutrons in an atom of Ti
(2) total mass of all the protons, neutrons, and electrons in an atom of Ti
(3) weighted average mass of the most abundant isotope of Ti
(4) weighted average mass of all the naturally occurring isotopes of Ti | 4 | atomic mass is the weighted average mass of the naturally occurring isotopes of an element |
5 An atom of which element has the largest atomic radius?
(1) Fe (3) Si
(2) Mg (4) Zn | 2 | Table S |
6 Which element requires the least amount of energy to remove the most loosely held electron from a gaseous atom in the ground state?
(1) bromine (3) sodium
(2) calcium (4) silver | 3 | Definition of Ionization energy _Table S |
7 A balanced equation representing a chemical reaction can be written using
(1) chemical formulas and mass numbers
(2) chemical formulas and coefficients
(3) first ionization energies and mass numbers
(4) first ionization energies and coefficients | 2 | That is what a reaction contains |
8 Every water molecule has two hydrogen atoms bonded to one oxygen atom. This fact supports the concept that elements in a compound are
(1) chemically combined in a fixed proportion
(2) chemically combined in proportions that vary
(3) physically mixed in a fixed proportion
(4) physically mixed in proportions that vary | 1 | fixed ratios |
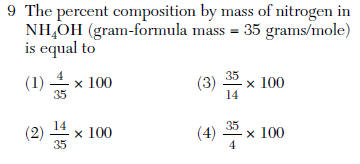 | 2 | (part /total ) x 100 |
10 Which Group 15 element exists as diatomic molecules at STP?
(1) phosphorus (3) bismuth
(2) nitrogen (4) arsenic | 2 | BrINClHOF |