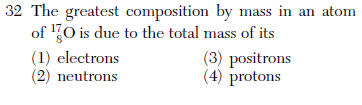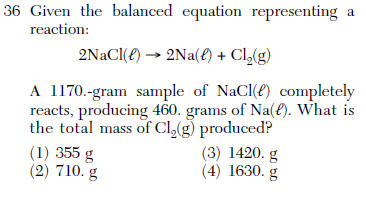Questions | Answer | Explanations |
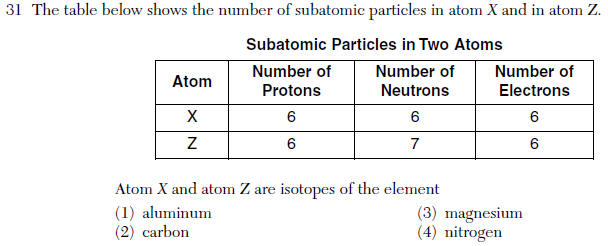 | 2 | Isotopes- same number of protons different number of neutrons |
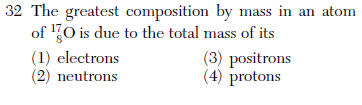 | 2 | there are more neutrons in O-17 neutrons 9 protons 8 |
33 The bond between which two atoms is most polar?
(1) Br and Cl (3) I and Cl
(2) Br and F (4) I and F | 4 | find the largest electronegativity difference Table S bonds=bend= Bonds compare ElectroNegativity Differences |
34 In the formula X2(SO4)3, the X represents a metal. This metal could be located on the
Periodic Table in
(1) Group 1 (3) Group 13
(2) Group 2 (4) Group 14 | 3 | SO4 has a charge of 2- and there are 3 of them=-6 X must have a charge of +3 (you only have 2 of them) Group 13 |
35 At STP, which element is solid, brittle, and a poor conductor of electricity?
(1) Al (3) Ne
(2) K (4) S | 4 | the nonmetal, not Ne it is a gas |
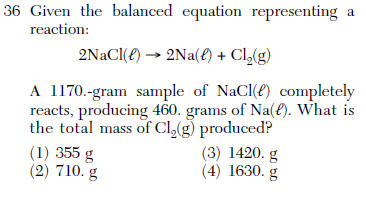 | 2 | conservation of mass what goes in must come out. Subtract. |
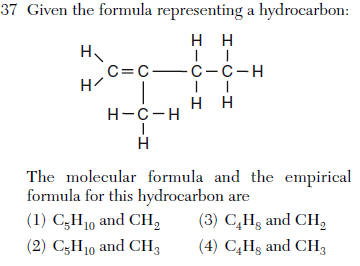 | 1 | count the atoms=molecular reduce to lowest whole number ratio=empirical |
38 Which element forms an ionic compound when it reacts with lithium?
(1) K (3) Kr
(2) Fe (4) Br | 4 | the nonmetal, not Kr it doesn't react |
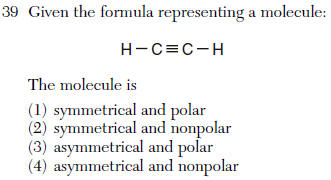 | 2 | SNAP=symmetrical is nonpolar, asymmetrical is polar |
40 Which compound has both ionic and covalent bonds?
(1) CO2 (3) NaI
(2) CH3OH (4) Na2CO3 | 4 | need a metal for it to be ionic and 2 nonmetals for it to be covalent |