Questions
Base your answers to questions 64 through 66 on the information below.
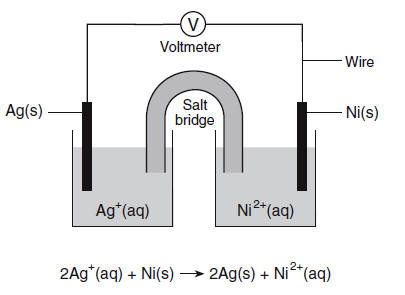
The diagram below represents an operating voltaic cell at 298 K and 1.0 atmosphere in a laboratory investigation. The reaction occurring in the cell is represented by the balanced ionic equation below.
64 Identify the anode in this cell. [1] HIGHLIGHT TO SEE THE ANSWER
65 Determine the total number of moles of Ni2+(aq) ions produced when 4.0 moles of Ag+(aq) ions completely react in this cell. [1] HIGHLIGHT TO SEE THE ANSWER
66 Write a balanced half-reaction equation for the reduction that occurs in this cell. [1] HIGHLIGHT TO SEE THE ANSWER
|