Questions
Base your answers to questions 75 through 78 on the information below.
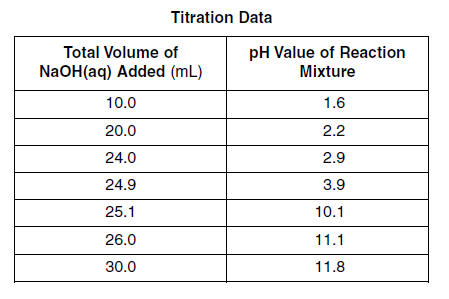
In one trial of an investigation, 50.0 milliliters of HCl(aq) of an unknown concentration is titrated with 0.10 M NaOH(aq). During the titration, the total volume of NaOH(aq) added and the corresponding pH value of the reaction mixture are measured and recorded in the table below.
75 On the grid in your answer booklet, plot the data from the table. Circle and connect the points. [1] 76 Determine the total volume of NaOH(aq) added when the reaction mixture has a pH value of 7.0. [1] HIGHLIGHT TO SEE THE ANSWER
77 Write a balanced equation that represents this neutralization reaction. [1] HIGHLIGHT TO SEE THE ANSWER
78 In another trial, 40.0 milliliters of HCl(aq) is completely neutralized by 20.0 milliliters of this 0.10 M NaOH(aq). Calculate the molarity of the titrated acid in this trial. Your response must include both a numerical setup and the calculated result. [2]
|