Questions
51
In your answer booklet, write an electron configuration for a silicon atom in an excited state. [1]Highlight box for Answer
2-7-5 1-8-5 2-8-3-1 and others |
Base your answers to questions 52 and 53 on the information below.
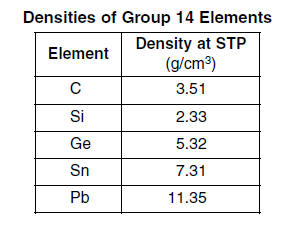
52 Identify one element from this table for each type of element: metal, metalloid, and nonmetal. [1]
Highlight box for Answer
| Metal --> | Sn or Pb | metalloid --> | Si or Ge | nonmetal--> | C |
53 Calculate the volume of a tin block that has a mass of 95.04 grams at STP. Your response must include both a numerical setup and the calculated result. [2]
Highlight box for Answers
7.31 g/cm 3 =95.04 g / V or 95.04/7.31 (1 point)Volume= 13.0 cm3 (1 point) Sig. Figs are not penalized here |