Questions | Answer | Explanations |
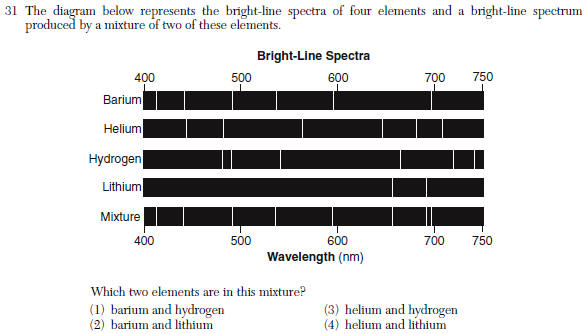 | 2 | match up the lines |
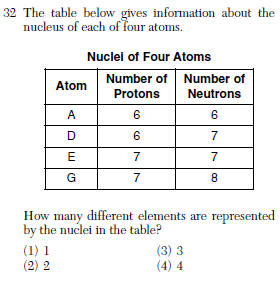 | 2 | protons determine the element |
33 What is the total number of valence electrons in an atom of germanium in the ground state?
(1) 8 (3) 14
(2) 2 (4) 4 | 4 | Ge outer electron shell 2-8-18-4 |
34 In the formula X2O5, the symbol X could represent an element in Group
(1) 1 (3) 15
(2) 2 (4) 18 | 3 | charge of X =+5 group 15 |
35 A 50.0-gram block of copper at 10.0°C is carefully lowered into 100.0 grams of water at 90.0°C in an insulated container. Which statement describes the transfer of heat in this system?
(1) The water loses heat to the block until both are at 10.0°C.
(2) The block gains heat from the water until both are at 90.0°C.
(3) The water loses heat and the block gains heat until both are at the same temperature that is between 10.0°C and 90.0°C.
(4) The water gains heat and the block loses heat until both are at the same temperature that is between 10.0°C and 90.0°C. | 3 | heat flows from hot to cold and the temperature of both end up between the two |
36 The compounds C2H4 and C4H8 have the same
(1) freezing point at standard pressure
(2) boiling point at standard pressure
(3) molecular formula
(4) empirical formula | 4 | they reduce to CH2 |
37 The chemical bond between which two atoms is most polar?
(1) C–N (3) S–Cl
(2) H–H (4) Si–O | 4 | look up their electronegativities. The biggest difference. |
38 What is the total amount of heat absorbed by 100.0 grams of water when the temperature of the water is increased from 30.0°C to 45.0°C?
(1) 418 J (3) 12 500 J
(2) 6270 J (4) 18 800 J | 2 | q=mc delta T= 100g x 4.18 x 15C |
39 Which process is exothermic?
(1) boiling of water
(2) melting of copper
(3) condensation of ethanol vapor
(4) sublimation of iodine | 3 | SLG problem g-->L |
40 Which sample, when dissolved in 1.0 liter of water, produces a solution with the lowest freezing point?
(1) 0.1 mol of C2H5OH
(2) 0.1 mol of LiBr
(3) 0.2 mol of C6H12O6
(4) 0.2 mol of CaCl2 | 4 | the most concentrated solution based on the ions 0.2m CaCl2 has 0.6 mol of ions |

