Question 69
Base your answers to questions 69 through 71 on the information below.
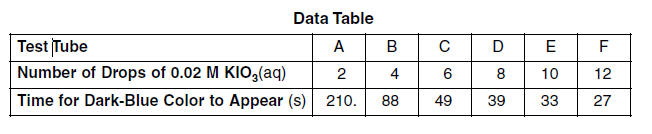
At room temperature, a reaction occurs when KIO3(aq) is mixed with NaHSO3(aq) that contains a small amount of starch. The colorless reaction mixture turns dark blue after a period of time that depends on the concentration of the reactants. In a laboratory, 12 drops of a 0.02 M NaHSO3(aq) solution containing starch were placed in each of six test tubes. A different number of drops of 0.02 M KIO3(aq) and enough water to maintain a constant volume were added to each test tube and the time for the dark-blue color to appear was measured. The data were recorded in the table below.
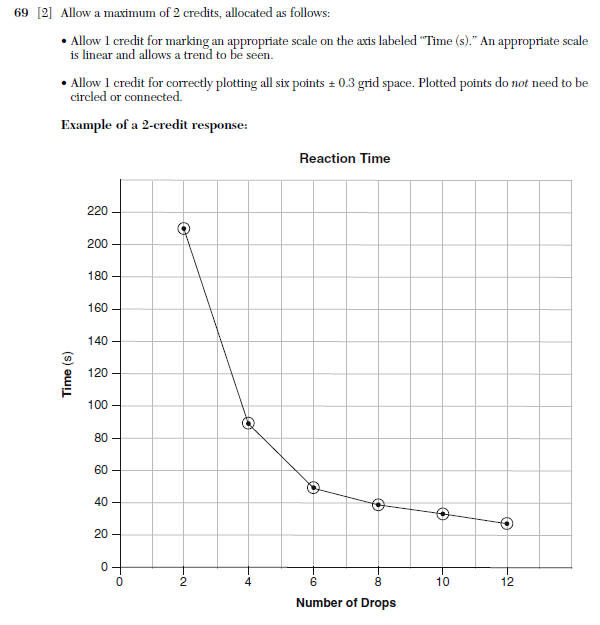
69 On the grid in your answer booklet: • Mark an appropriate scale on the axis labeled “Time (s).” [1] • Plot the data from the data table. Circle and connect the points. [1]
|
Back to Questions 69-71