A student constructs an electrochemical cell during a laboratory investigation. When the switch is closed, electrons now through the external circuit. The diagram and equation below represent this
cell and the reaction that occurs.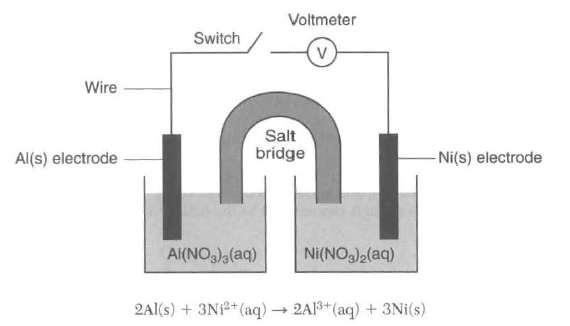
72
State the direction of electron flow through the wire when the switch is closed. [1]Highlight box to reveal answer
from Al to Ni or to the right through the wire (Top of table J to the bottom) |
73 Write a balanced halfˇ reaction equation for the oxidation that occurs when the switch is closed. [1]
Highlight box to reveal answer
Al ==> Al3+ + 3e- or 2Al ==> 2Al3+ + 6e- |
74 Determine the number of moles of Al(s) needed to completely react with 9.0 moles of Ni
2+(aq) ions. [1]Highlight box to reveal answer
6.0moles WORK==> x/2Al = 9.0mols /3Ni2+ |
75 State, in terms of energy, why this cell is a voltaic cell.
[I]Highlight box to reveal answer
it is a spontaneous reaction that converts chemical to electrical energy or A battery is not required for the cell to operate...+ many more |
on to Questions 76-78