Questions
Explanations
(1) molarity (2) parts per million
(3) percent by mass (4) percent by volume
(1) chemical energy (2) electrical energy (3) nuclear energy (4) thermal energy
avg. KE is temperature (thermal)
(1) total kinetic energy of the particles in the sample
(2) total potential energy of the particles in the sample
(3) average potential energy of the particles in the sample
(4) average kinetic energy of the particles in the sample
(1) mole (2) joule (3) kelvin (4) pascal
(1) Br2(l) (2) Cl2(g) (3) CO2(S) (4) SO2(aq)
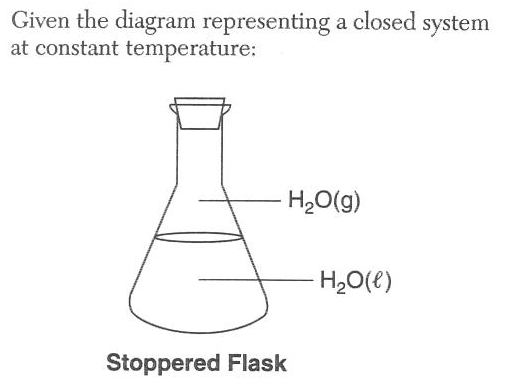
Which statement describes this system at equilibrium?
(1) The mass of H2O(l) equals the mass of H2O(g)
(2) The volume of H2O(l) equals the volume of H2O(g)
(3) The number of moles of H2O(l) equals the number of moles of H2O(g)
(4) The rate of evaporation of H2O(l) equals the rate of condensation of H2O(g)
Con Con Requal
concentrations constant rates are equal
(1) combustion (2) neutralization (3) oxidation (4) reduction
(1) CH3CHO (2) CH3CH2OH (3) CH3COOH (4) CH3OCH3
CH3COOH
(1) less mass and greater penetrating power
(2) less mass and less penetrating power
(3) more mass and greater penetrating power
(4) more mass and less penetrating power
helium is stopped easier than an electron
(1) charge (2) energy (3) isomers (4) volume
E=mc2