Questions
Explanations
(1) ethanoic acid (2) ethanol (3) propanone (4) water
(1) 393.5 kJ (2) 837.8 kJ (3) 1676 kJ (4) 3351 kJ
delta H =-3351kJ
this reaction is for 2 moles, you need 1 mole, so half the amount of energy
![]()
What
occurred as a result of this reaction?(1)
Energy was absorbed, and entropy increased.(2)
Energy was absorbed, and entropy, decreased(3) Energy
was released, and entropy increased(4)
Energy was released, and entropy decreased.
entropy is randomness, which increases... liquid to gases
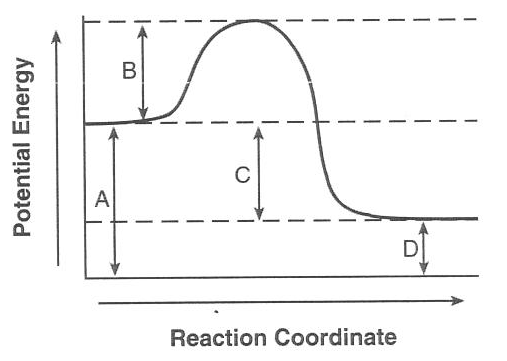
The activation energy
for the reverse reaction is represented by(1) A + B (2) B + C (3) B + D (4) C + D
B+C
it can start from the right
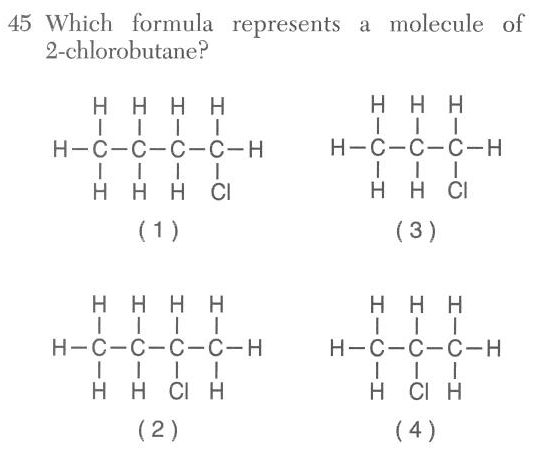
46 'Which formula represents an unsaturated hydrocarbon?
(1) CH4 (2) C2H4 (3) C3H8 (4) C4H10
unsaturated is not an alkane
not CnH2n+2
47 Which ion is most easily reduced?
(l )
Zn2+ (2) Mg2+ (3) Co2+ (4) Ca2+Oxidation is on top
48 Given the balanced equation representing a reaction:
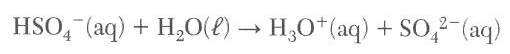
According to one acid-base theory, the H2O(l) molecules act as
(1) a base because they accept H
+ ions(2) a base because they donate H+ ions
(3)
an acid because they accept H + ions(4) an acid because they donate 1-1+ ions
it acts as a base
49 Which equation represents an oxidation reduction reaction?
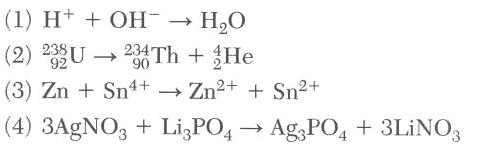
or find the free element
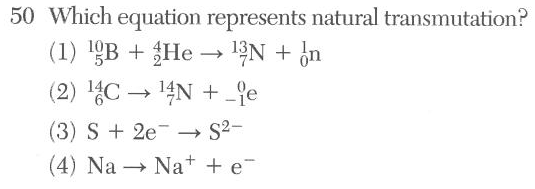
artificial 2 reactants