Questions
Explanations
there are 8 electrons so 8 protons
(1)2-8-18-6 (2) 2-8-18-7 (3) 2-8-17-7 (4) 2-8-17-8
promoted an electron from the 3rd energy level to the 4th.
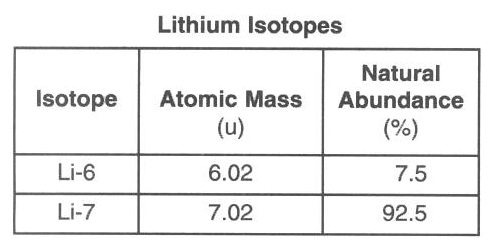
Which numerical setup can be used to determine the atomic mass of lithium?
(1) (0.075)(6.02u) + (0.925)(7.02u)
(2) (0.925)(6.02u) + (0.075)(7.02u)
(3) (7.5)(6.02u) + (92.5)(7.02u)
(4) (92.5)(6.02u) + (7.5)(7.02u)
mass x percent (as a decimal) + mass x percent (as a decimal)
(1) Group 1 (2) Group 2 (3) Group 13 (4) Group 15
X will be in group 2
(1) increasing atomic radius (2) increasing electronegativity
(3) decreasing atomic mass (4) decreasing first ionization energy
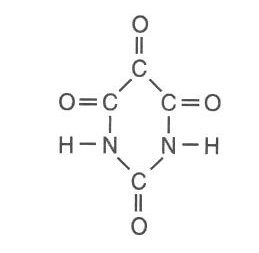
Which molecular formula and empirical formula represent this compound?
(1) C2HNO2 and CHNO (2) C2HNO2 and C2HNO2
(3) C4H2N2O4 and CHNO (4) C4H2N2O4 and C2HNO2
find the lowest ratio of atoms for the empirical formula
(1) 112g/mol (2) 121g/mol (3) 149g/mol (4) 242g/mol
(1) C (2) V (3) Ne (4)Sb
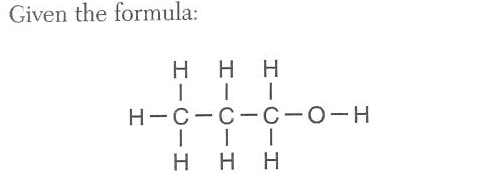
The bonds between which two atoms has the greatest degree of polarity?
(1) C and C (2) C and O (3) H and C (4) H and O
Large the difference the more polar the bond
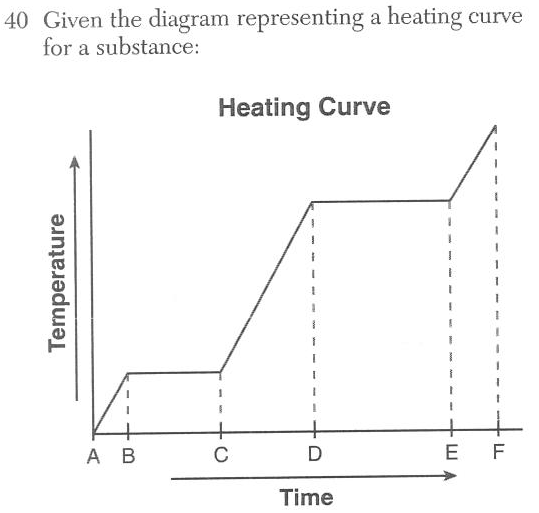
During which time interval is the average kinetic energy of the particles of the substance constant while the potential energy of the particles increases?
(1) AC (2) BC (3) CD (4)DF