Questions | Answer | Links | Explanations |
| 1 The mass of the proton is approximately equal to the mass of (1)an alpha particle (2) a beta particle (3) a positron (4) a neutron | 4 | link | Protons and neutrons have the same mass |
| 2 An orbital of an atom is defined as the most probable location of (1) an electron (2) a neutron (3) a positron (4) a proton | 1 | link | electrons reside in orbitals |
| 3 What must occur when an electron in an atom returns from a higher energy state to a lower energy state? (1) A specific amount of energy is released (2) A random amount of energy is released (3) The atom undergoes transmutation (4) The atom spontaneously decays | 1 | link | light is emitted at specific wavelengths and therefore specific amounts of energy |
|
| 4 Which element is liquid at 305K and 1.0 atm? (1) magnesium (2) fluorine (3) gallium (4) iodine | 3 | link | Using table S, the melting point needs to be lower than 305K and the boiling point needs to be greater than 305K |
| 5 Which list of elements consist of a metal, a metalloid and a nonmetal? (1) Li, Na, Rb (2) Cr, Mo, W (3) Sn, Si, C (4) O, S, Te | 3 | link | metals are on the left of the PT, Nonmetals on the right, and metalloids are on the staircase |
| 6 At STP, which physical property of aluminum always remains the same from sample to sample? (1) mass (2) density (3) length (4) volume | 2 | link | density is a proportion of mass to volume and will not change |
|
| 7 Which describes a chemical property of silicon? (1) Silicon has a blue-gray color (2) Silicon is a brittle solid at 20oC (3) Silicon melts at 1414oC (4) Silicon reacts with fluorine | 4 | link | reactions are chemical properties, the others are physical properties |
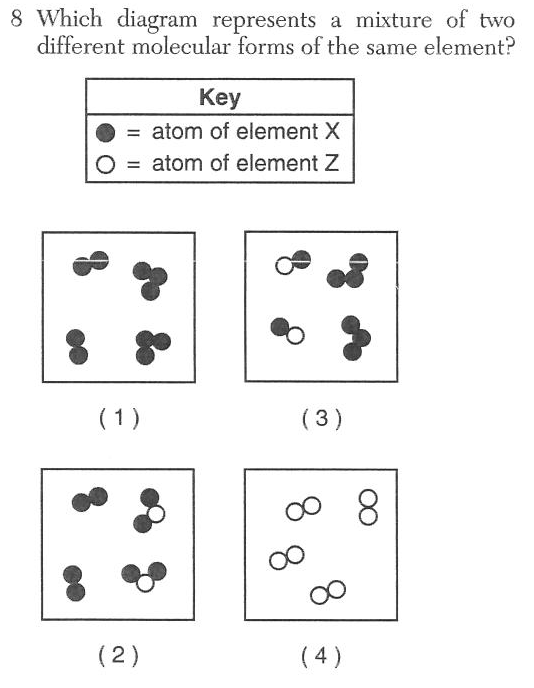 | 1 | link | Elements are made of only one type of atom, the mixture has diatomic and triatomic molecules together |
|
| 9 A compound is broken down by chemical means during (1) chromatography (2) distillation (3) electrolysis (4) filtration | 3 | link | electrolysis separates compounds into its elements by using electricity |
| 10 Which quantities must be conserved in all chemical reactions? (1) mass, charge, density (2) mass, charge, energy (3) charge volume, density (4) charge volume, energy | 2 | link | mass, charge and energy are all conserved |
