Questions
Explanations
31 The diagram below represents the bright-line spectra of four elements and a bright-line spectrum produced by a mixture of three of these elements.
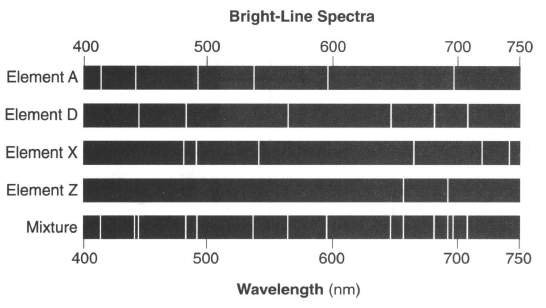
Which element is not present in the mixture?
(1) A (2) D (3) X (4) Z
if one is missing it is out
32 What is the overall charge of an ion that has 12 protons, 10 electrons, and 14 neutrons?
(1) 2- (3) 4
(2) 2+ (4) 4+
electrons are 1-
Neutrons are zero
add them up
33 As the elements in Period 3 are considered in order of increasing atomic number, there is a general decrease in
(1) atomic mass (2) atomic radius
(3) electronegativity (4) first ionization energy
atomic radius decreases
See table S
34 Which electron configuration represents the electrons of a sulfur atom in an excited state?
(1) 2-6-6 (3) 2-8-4
(2) 2-7-7 (4) 2-8-6
NOT 2-8-6 that is the ground state configuration
35 Given the word equation:
sodium chlorate ==> sodium chloride + oxygen
Which type of chemical reaction is represented by this equation?
(1) double replacement (3) decomposition
(2) single replacement (4) synthesis
AX--> A + X
one reactant
36 Which compound has the highest percent composition by mass of strontium?
(1) SrC12 (2) SrI2 (3) SrO (4) SrS
the highest percent is the compound that has the smallest formula mass
37 Given the formula for hydrazine:
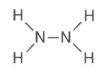
How many pairs of electrons are shared between the two nitrogen atoms?
(1) 1 (3) 3
(2) 2 (4) 4
or 1 PAIR of electrons
38 Which formulas represent one ionic compound and one molecular compound?
(1) N2 and SO2 (2) Cl2 and H2S
(3) BaCl2 and N2O4(4) NaOH and BaSO4
2 or more nonmetals atoms bonded
(1) -73 K (2) 73 K (3) 200. K (4) 473 K
Back page of RT
A 10.0-gram sample of H2O(l) at 23.0°C absorbs 209 joules of heat. What is the final temperature of the H2O(l) sample?
(1) 5.0°C (3) 28.0°C
(2) 18.0°C (4) 50.0°C
DT=mC/Q=10g(4.18)/209J= 5°C
it was at 23, so add 5 to that because it absorbed energy