Questions
Explanations
41 Given the equation representing a system at equilibrium:
![]()
When the concentration of Cl-(aq) is increased, the concentration of Ag+(aq)
(1) decreases, and the amount of AgCl(s) increases
(2) decreases, and the amount of AgCl(s) decreases
(3) increases, and the amount of AgCl(s) increases
(4) increases, and the amount of AgCl(s) decreases
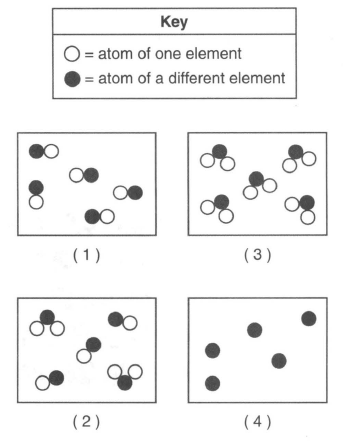
42 Which particle diagram represents a sample of matter that can not be broken down by chemical means?
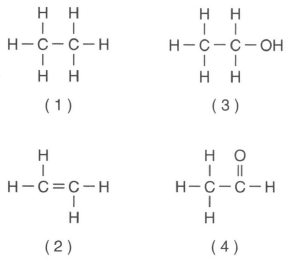
43 Which formula represents an unsaturated hydrocarbon?
44 When the pH of a solution is changed from 4 to 3, the hydronium ion concentration of the solution
(1) decreases by a factor of 10
(2) increases by a factor of 10
(3) decreases by a factor of 100
(4) increases by a factor of 100
every pH unit change is a 10 fold change.
45 Three samples of the same solution are tested, each with a different indicator. All three indicators, bromthymol blue, bromcresol green, and thymol blue, appear blue
if the pH of the solution is(1)
4.7 (2) 6.0 (3) 7.8 (4) 9.946 A 10.0-milliliter sample of NaOH(aq) is neutralized by 40.0 milliliters of 0.50 M HCl. What is the molarity of the NaOH(aq)?
(1) 1.0 M (3) 0.25 M
(2) 2.0 M (4) 0.50 M
MaVa=MbVb
(0.50M)(40.0mL)=M(10.omL)
47 Radiation is spontaneously emitted from hydrogen-3 nuclei, but radiation is not spontaneously emitted from hydrogen-l nuclei or hydrogen-2 nuclei. Which hydrogen nuclei are stable?
(1) nuclei of H-l and H-2, only
(2) nuclei of H-l and H-3, only
(3) nuclei of H-2 and H-3, only
(4) nuclei of H-l, H-2, and H-3
48 Given the equation representing a nuclear reaction in which X represents a nuclide:
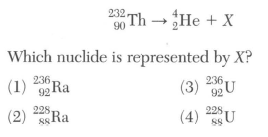
mass number 232=4-X
atomic number 90=2-Z Z will determine the new symbol, use the PT
49 After decaying for 48 hours,
l/16 of the original mass of a radioisotope sample remains unchanged. What is the half-life of this radioisotope?(1) 3.0 h (3) 12 h
(2) 9.6 h (4) 24 h
4 half lives
48hours/4= 12 hrs/halflife
50 Which balanced equation represents nuclear fusion?
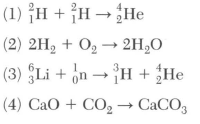
choices 2 and 4 are not nuclear