Cylinder A has a movable piston and contains hydrogen gas. An identical cylinder, B, contains methane gas. The diagram below represents these cylinders and the conditions of pressure, volume, and temperature of the gas in each cylinder.
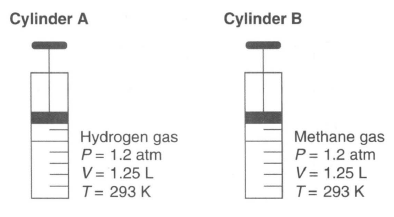
60 Compare the total number of gas molecules in cylinder A to the total number of gas molecules in cylinder B. [1]
Highlight box to reveal answer
The number of gas molecules in cylinder A is the same as the number of gas molecules in cylinder 13. |
61 State a change in temperature and a change in pressure that will cause the gas in cylinder A to behave more like an ideal gas. [1]
Highlight box to reveal answer
Temperate: higher Pressure: lower Temperature: above 293 K Pressure: below 1.2 atm |
62 In the space in your answer booklet, show a numerical setup for calculating the volume of the gas in cylinder B at STP. [1]
Highlight box to reveal answer
| (1.2atm)(1.25L)/293K=(1.0 atm)(V2)/273K or (273)(1.2)(1.25)/293 |