There are several isomers of C6H14. The formulas and boiling points for two of these isomers are given in the table below.
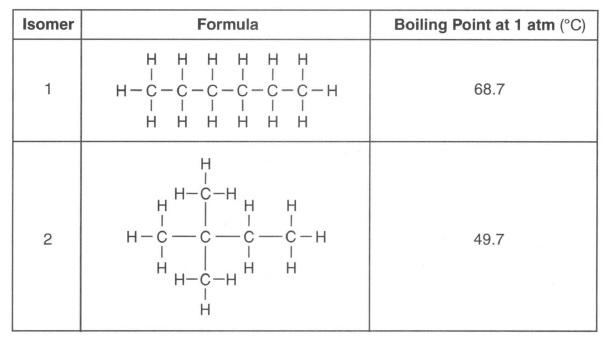
63 Identify the homologous series to which these isomers belong. [1]
Highlight box to reveal answer
| alkanes OR CnH2n+2 ( I have no idea why this 2nd one is allowed as an answer) |
64 Write the empirical formula for isomer 1. [1]
Highlight box to reveal answer
65 Explain, in terms of intermolecular forces, why isomer 2 boils at a lower temperature than isomer 1. [1]
Highlight box to reveal answer
Isomer 2 boils at a lower temperature because it has weaker intermolecular forces than isomer 1 .The intermolecular forces in isomer 1 are stronger. |