|
Questions | Answer | Links | Explanations |
| 36 Which reaction is single replacement? (1) 2H2O2 --> 2H2O + O2 (2) 2H2 + O2 --> 2H2O (3) H2SO4 + Mg --> H2 + MgSO4 (4) HCl + KOH --> KCl + H2O | 3 | link | an element replaces another element in a compound Choice #4 is the answer to #77 |
| 37 The accepted value for the percent by mass of water in a hydrate is 36.0%. In a laboratory activity, a student determined the percent by mass of water in the hydrate to be 37.8%. What is the percent error for the student's measured value? (1) 5.0% (3) 1.8% (2) 4.8% (4) 0.05% | 1 | | equation is on the back page, plug in numbers ((37.8-36.0)/36.0) x 100% |
38 The boiling points, at standard pressure, of four compounds are given in the table below.| Compound | Boiling Point (oC) | | H2O | 100.0 | | H2S | -59.6 | | H2Se | -41.3 | | H2Te | -2.0 |
What type of attraction can be used to explain the unusually high boiling point of H2O? (1) ionic bonding (2) hydrogen bonding (3) polar covalent bonding (4) nonpolar covalent bonding | 2 | link | H-bonding question look for H with F O or N |
|
| 39 Which formula represents a molecule with the most polar bond? (1) CO (3) HI (2) NO (4) HCl | 4 | link | largest difference in electronegativity is the most polar see table S of electronegativity values |
| 40 The graph below represents the uniform heating or a substance from the solid to the gas phase. 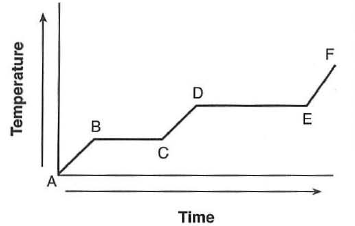
Which line segment of the graph represents boiling? (1) AB (3) CD (2) BC (4) DE | 4 | link | the upper plateau is boiling |
|
