Questions
Explanations
41 A 1-gram sample or a compound is added to 100 grams of H2O(l) and the resulting mixture is then thoroughly stirred. Some of the compound is then separated from the mixture by filtration. Based on Table F, the compound could be
(1) AgCl (3) NaCl
(2) CaCl2 (4) NiCl2
See Table F
FYI 3 are soluble, AgCl is insoluble
(1) 2.26 X 10 J (3) 2.26 X 103 J
(2) 2.26 X 102 J (4) 2.26 X 105 J
q= 100g x 2260J/g =226,000J
or 2.26 x 105J
(1) The mass of the sample decreases.
(2) The number of moles of gas increases.
(3) The volume of each atom decreases.
(4) The frequency of collisions between atoms increases.
2SO2(g) + O2(g) ==> 2SO3(g) + heat
Which change causes the equilibrium to shift to the right?
(1) adding a catalyst
(2) adding more O2(g)
(3) decreasing the pressure
(4) increasing the temperature
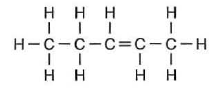
What is the chemical name of this compound?
(1) 2-pentene (3) 3-pentene
(2) 2-pentyne (4) 3-pentyne
You count from the right
2-pentene