Questions
Explanations
(1) +7 (3) +3
(2) +2 (4) +4
O is -2 x 4 so -8
Leaving Mn to be +7 so it all adds to 0
(1) decreased by a factor of 2
(2) decreased by a factor of 10
(3) increased by a factor of 2
(4) increased by a factor or 10
hydronium increases if the pH decreases (more acidic) and decreases as the pH increase (more basic)
(1) red (3) pink
(2) blue (4) yellow
thymol blue above 9.6 is blue
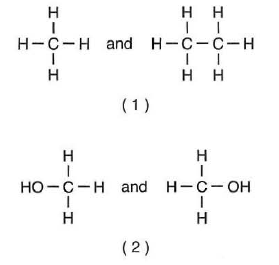
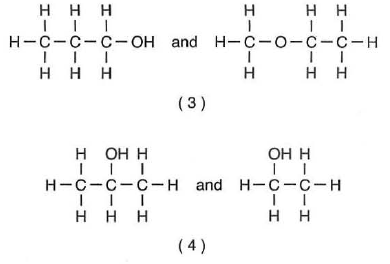
count the atoms, but is must be a different structure
(1) detection of disease
(2) neutralization of an acid spill
(3) decreasing the dissolved O2(g) level in seawater
(4) increasing the concentration of CO2(g) in the atmosphere