Questions
Explanations
(1) protons and electrons
(2) protons and neutrons
(3) protons and neutrons
(4) positrons and electrons
electrons outside
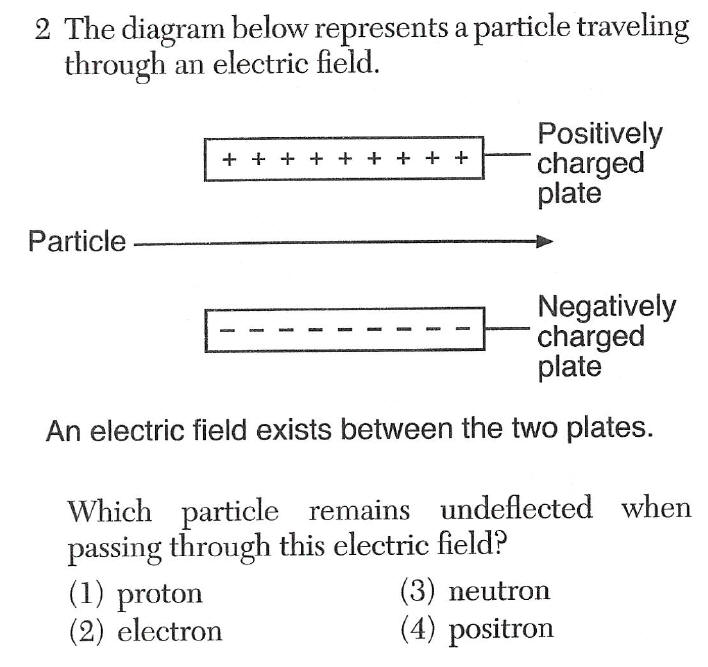
neutrons=no charge
(1) equal to the mass of a proton
(2) equal to the mass of a neutron
(3) greater than the mass of a proton
(4) less than the mass of a neutron
electrons much smaller mass
(1) first shell is lower
(2) first shell is the same
(3) third shell is lower
(4) third shell is the same
(1) sodium
(2) phosphorous
(3) nitrogen
(4) fluorine
last number
2-7 fluorine