Questions
Explanations
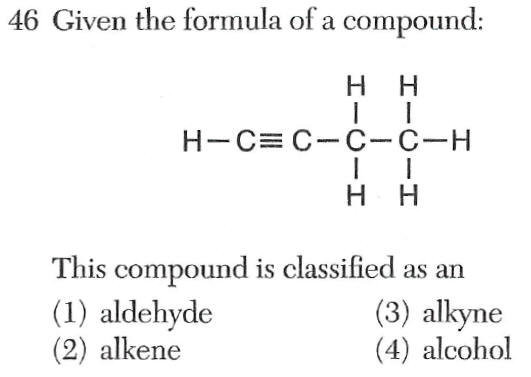
alkyne
Table Q
(1) C2H4 + H2O -> CH3CH2OH
(2) C2H4 +HCl --> CH3CH2Cl
(3) C6H12O6 --> 2CH3CH2OH + CO2
(4) 2CH3CHO --> C3H5CHO + H2O
3CuCl2 + 2Al --> 3 Cu + 2 AlCl3
The oxidation number of copper changes from
(1) +1 to 0
(2) +2 to 0
(3) +2 to + 1
(4) +6 to +3
+2 to 0
each Cl is -1
CH3COOH + H2O <=> CH3COO- + H3O+
According to one acid-base theory, the two H+ donors in the equation are:
(1) CH3COOH and H2O
(2) CH3COOH and H3O+
(3) CH3COO- and H2O
(4) CH3COO- and H3O+
look are the pairs that are off by 1 H
acids has that extra H
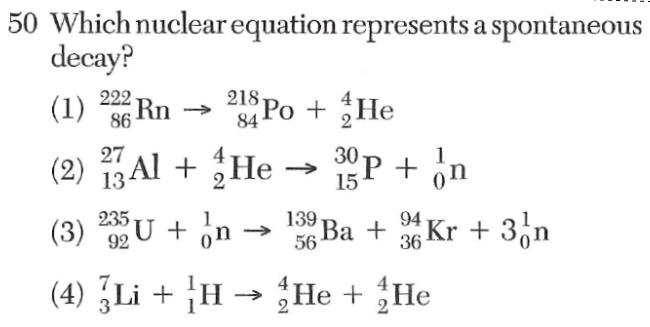
1 on the left of the arrow