|
Questions | Answer | Links | Explanations |
31The bright-line spectra of four elements, G,J, L, and
M, and a mixture of at least two of these elements are given below.
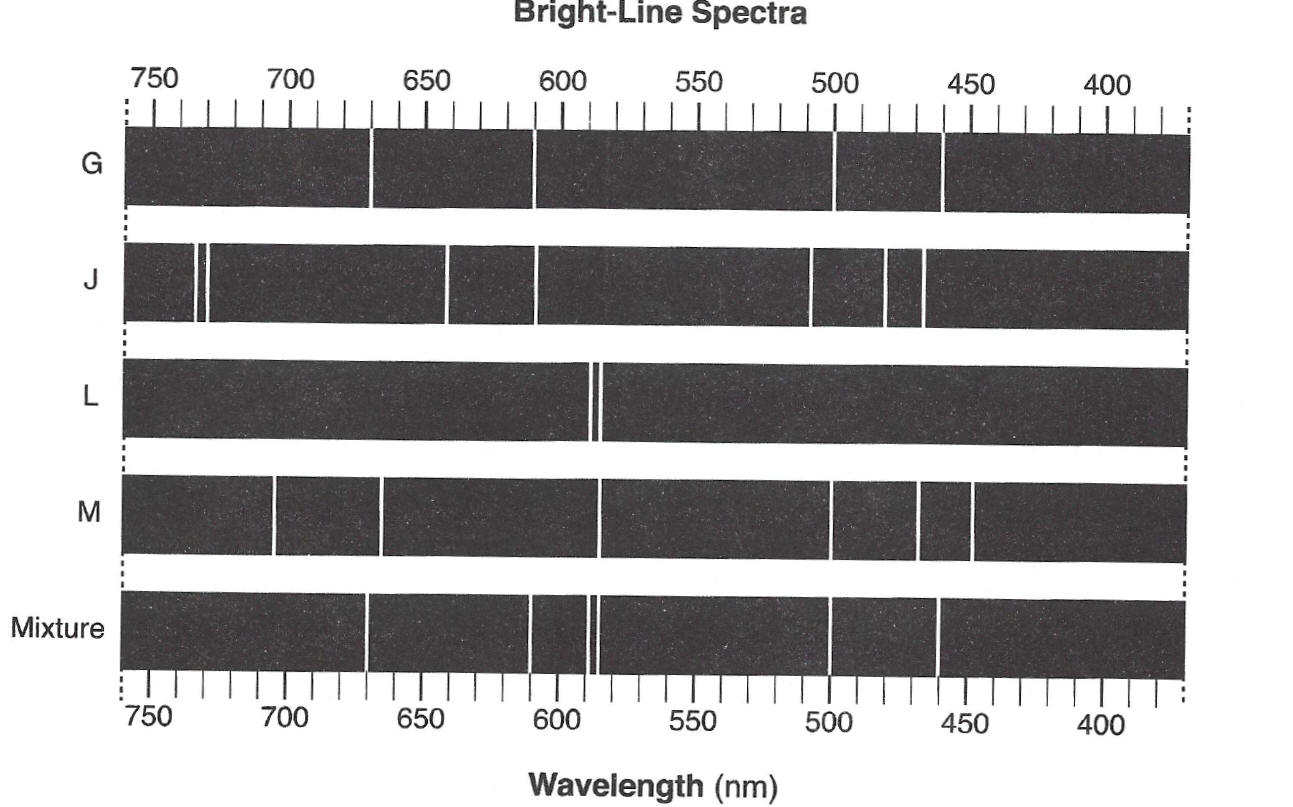
Which elements are present in the mixture?
(1) G and J
(2) G and L
(3) M, J, and G
(4) M, J, and L
|
2 | |
line up the lines...all lines must be there |
| 32 Which electron configuration represents an atom of
chlorine in an excite state? (1) 2-8-7-2
(2) 2-8-7
(3) 2-8-8
(4) 2-7-8
|
4 | |
must have 17 electrons and not be
2-8-7...move one up
2-7-8 |
| 33 A student measures the mass and volume of a sample of
aluminum at room temperature, and calculates the density of Al to be
2.85 grams per cubic centimeter. Based on Table S, what is the percent
error for the student's calculated density of Al? (1) 2.7%
(2) 5.3%
(3) 5.6%
(4) 95%
|
3 | |
2.70 on table S is accepted value
plug in
(2.85 -2.70)/2.70 x100# |
|
| 34 Magnesium and calcium have similar properties because
their atoms in the ground state have (1) equal numbers of
protons and electrons
(2) equal numbers of protons and neutrons
(3) two electrons in the first shell
(4) two electrons in the outermost shell
|
4 | |
similar properties same number of valence
electrons...outer shell |
| 35 As the elements in Period 2 of the Periodic Table are
considered in order from left to right, which property generally
decreases? (1) atomic radius
(2) electronegativity
(3) ionization energy
(4) nuclear charge
|
1 | |
use table S
snowman
Oo. |
|
