|
62. Explain in terms of BOTH protons and neutrons,
why Co-59 and Co-60 are isotopes of cobalt.
|
Answer--> same number of protons, different number of neutrons |
63. Compare the penetrating power of the beta and
gamma radiation.
| Answer--> gamma is more
penetrating than beta or beta is less penetrating than gamma |
64. Complete the nuclear equation in your
answer book
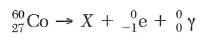
65. Based on Table N, determine
the total time required for a 80.00 gram sample of Co-60 to decay
until only 10.00 grams of the sample remains unchanged.|
Answer--> 15.813y 80g--> 40g--> 20g--> 10g
3 x 5.271y |
F halides |