|
Questions | Answer | Links | Explanations |
|
36 Given the balanced equation for the reaction between butane and oxygen:
2C4H10 + 13O2 --> 8CO2 + 10 H2O
+ Energy
How many moles of carbon dioxide are produced when 5.0 moles of butane
react completely?
(1) 5.0 mol
(2) 10. mol
(3) 20. mol
(4) 40. mol
| 3 | |
set up a proportion
5moles is to 2 C4H10
as X moles is to 8 CO2 |
|
37 What ios the percent composition by mass of nitrogen in the compound
N2H4 (gram-formula mass = 32 g/mol).
(1) 13%
(2) 44%
(3) 88%
(4) 93%
|
3 | |
n is 14 but there are 2
28/32 x 100 |
|
38 Which ion in the ground state has the same electron configuration as
an atom of neon in the ground state?
(1) Ca2+
(2) Cl-
(3) Li+
(4) O2-
|
4 | |
so 10 electrons
negative charge has more electrons
positive gave them away |
|
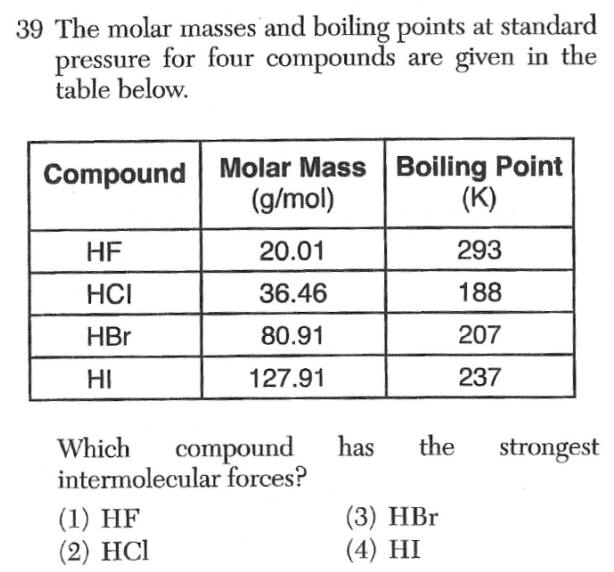 |
1 | |
Hydrogen Bonding question
H with F,O or N has teh strongest forces |
|

|
1 | |
Xenon is a gas at STP |
|

