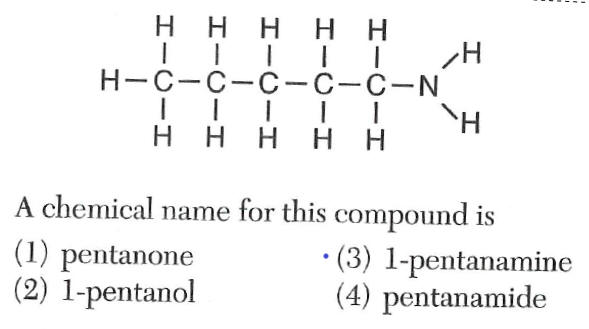
Questions
Explanations
(1) 1100J
(2) 4700J
(3) 6300J
(4) 11000J
Q=75g x 4.18 x (35-20)
(1) 10. mL of 1 M HCl(aq) at 10oC
(2) 10. mL of 1 M HCl(aq) at 25oC
(3) 10. mL of 3 M HCl(aq) at 10oC
(4) 10. mL of 3 M HCl(aq) at 25oC
highest temp
N2O4(g) <=> 2NO2(g)
Which statement describes the concentration of the two gases in this system
(1) The concentration of N2O4(g) must be less than the concentration of NO2(g)
(2) The concentration of N2O4(g) must be greater than the concentration of NO2(g)
(3) The concentration of N2O4(g) and the concentration of NO2(g) must be equal.
(4) The concentration of N2O4(g) and the concentration of NO2(g) must be constant.
Concentrations constant
rates are equal
PCl5(g) + energy <=> PCl3(g) + Cl2(g)
Which change will cause the equilibrium to shift to the right?
(1) adding a catalyst
(2) adding more PCl3(g)
(3) increasing the pressure
(4) increasing the temperature
45