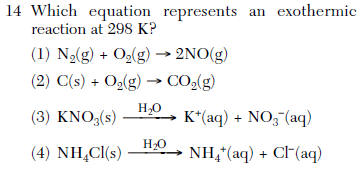Questions | Answer | Explanations |
11 The compound XCl is classified as ionic if X represents the element
(1) H (3) Rb
(2) I (4) Br | 3 | has a 1+ charge, Group 1 |
12 The chemical bonding in sodium phosphate, Na3PO4, is classified as
(1) ionic, only
(2) metallic, only
(3) both covalent and ionic
(4) both covalent and metallic | 3 | 3 elements bonded and one is a metal both Ionic and Covalent |
13 Which element is composed of molecules that each contain a multiple covalent bond?
(1) chlorine (3) hydrogen
(2) fluorine (4) nitrogen | 4 | nitrogen bonds 3 times the others only bond once |
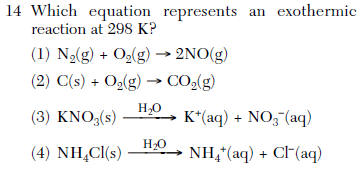 | 2 | Use TABLE I negative DH is exothermic |
15 Standard pressure is equal to
(1) 1 atm (3) 273 atm
(2) 1 kPa (4) 273 kPa | 1 | see TABLE A |
16 A large sample of solid calcium sulfate is crushed into smaller pieces for testing. Which two physical properties are the same for both the large sample and one of the smaller pieces?
(1) mass and density
(2) mass and volume
(3) solubility and density
(4) solubility and volume | 3 | properties that will not change |
17 According to the kinetic molecular theory, the molecules of an ideal gas
(1) have a strong attraction for each other
(2) have significant volume
(3) move in random, constant, straight-line motion
(4) are closely packed in a regular repeating pattern | 3 | Rules of an Ideal Gas random, straight-line motion no attractions |
18 At 65°C, which compound has a vapor pressure of 58 kilopascals?
(1) ethanoic acid (3) propanone
(2) ethanol (4) water | 2 | use TABLE H |
19 At STP, which 2.0-gram sample of matter uniformly fills a 340-milliliter closed container?
(1) Br2(l) (3) KCl(aq)
(2) Fe(NO3)2(s) (4) Xe(g) | 4 | Gas ==>uniformly fills |
| 20 Compared to the freezing point and boiling point of water at 1 atmosphere, a solution of a salt and water at 1 atmosphere has a (1) lower freezing point and a lower boiling point
(2) lower freezing point and a higher boiling point
(3) higher freezing point and a lower boiling point
(4) higher freezing point and a higher boiling point | 2 | BP increase FP decrease |