Questions | Answer | Explanations |
31 Which electron configuration represents an atom in an excited state?
(1) 2–7 (3) 2–8–1
(2) 2–6–2 (4) 2–8–8–2 | 2 | 2 should be 2-8 |
32 Magnesium and calcium have similar chemical properties because a magnesium atom and a calcium atom have the same
(1) atomic number
(2) mass number
(3) total number of electron shells
(4) total number of valence electrons | 4 | valance electrons are the same |
33 What is the empirical formula for a compound
with the molecular formula C6H12Cl2O2?
(1) CHClO (3) C3H6ClO
(2) CH2ClO (4) C6H12Cl2O2 | 3 | reduce the formula |
34 Given the balanced equation representing a reaction:
4Al(s) + 3O2(g)-->2Al2O3(s)
Which type of chemical reaction is represented by this equation?
(1) double replacement
(2) single replacement
(3) substitution
(4) synthesis | 4 | A + B =>AB |
35 A person with a body temperature of 37°C holds an ice cube with a temperature of 0°C in a room where the air temperature is 20.°C. The direction of heat flow is
(1) from the person to the ice, only
(2) from the person to the ice and air, and from the air to the ice
(3) from the ice to the person, only
(4) from the ice to the person and air, and from the air to the person | 2 | hot => cold |
36 What is the total mass of solute in 1000. grams of a solution having a concentration of 5 parts per million?
(1) 0.005 g (3) 0.5 g
(2) 0.05 g (4) 5 g | 1 | MATH use ppm equation on back of the RT |
37 Which compound is least soluble in water at 60.°C?
(1) KClO3 (3) NaCl
(2) KNO3 (4) NH4Cl | 1 | lowest on table G at 60.°C |
38 At standard pressure, which element has a freezing point below standard temperature?
(1) In (3) Hf
(2) Ir (4) Hg | 4 | Mercury Hg Table S |
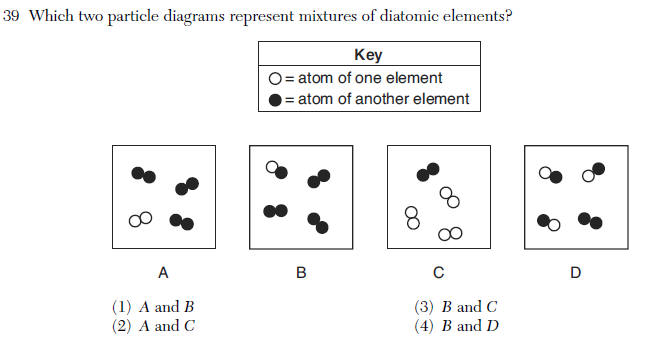 | 2 | mixture 2 substances separated diatomic 2 identical elements bonded |
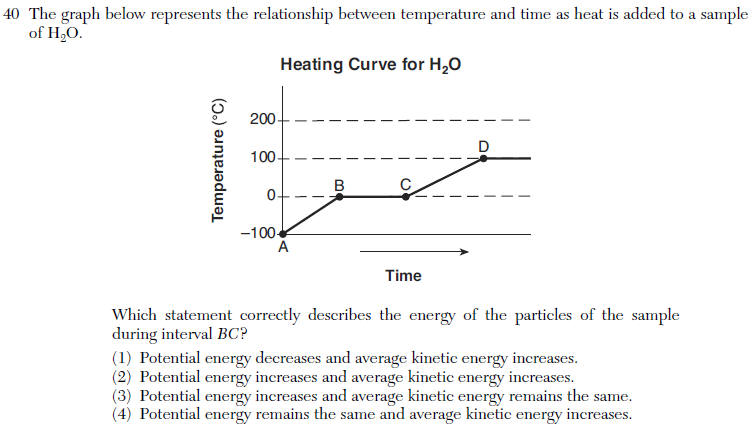 | 3 | plateau=phase change=PE change Ke remains the same |

