Questions
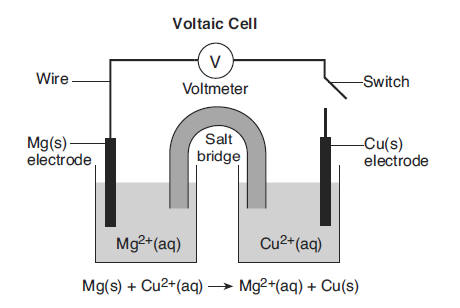
A voltaic cell with magnesium and copper electrodes is shown in the diagram below. The copper electrode has a mass of 15.0 grams.
When the switch is closed, the reaction in the cell begins. The balanced ionic equation for the reaction in the cell is shown below the cell diagram. After several hours, the copper electrode is removed, rinsed with water, and dried. At this time, the mass of the copper electrode is greater than 15.0 grams. 60 State the direction of electron flow through the wire between the electrodes when the switch is closed. [1] HIGHLIGHT TO SEE THE ANSWER
61 State the purpose of the salt bridge in this cell. [1] HIGHLIGHT TO SEE THE ANSWER
62 Explain, in terms of copper ions and copper atoms, why the mass of the copper electrode increases as the cell operates. Your response must include information about both copper ions and copper atoms. [1] HIGHLIGHT TO SEE THE ANSWER
|