Questions
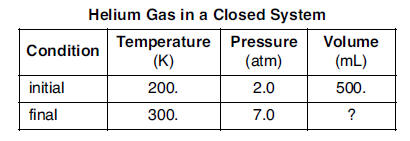
A sample of helium gas is in a closed system with a movable piston. The volume of the gas sample is changed when both the temperature and the pressure of the sample are increased. The table below shows the initial temperature, pressure, and volume of the gas sample, as well as the final temperature and pressure of the sample.
51 In the space in your answer booklet, show a correct numerical setup for calculating the final volume of the helium gas sample. [1]52 Convert the final temperature of the helium gas sample to degrees Celsius. [ 1]HIGHLIGHT TO SEE THE ANSWER
53 Compare the total number of gas particles in the sample under the initial conditions to the total number of gas particles in the sample under the final conditions. [ 1]HIGHLIGHT TO SEE THE ANSWER
|