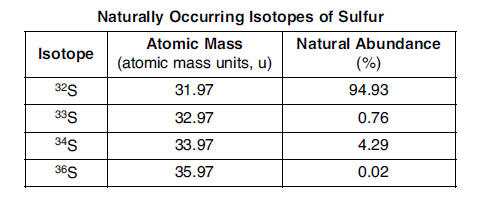
63 State, in terms of the number of subatomic particles, one similarity and one difference between the atoms of these isotopes of sulfur. [1]
HIGHLIGHT TO SEE THE ANSWER
Similarity: All atoms of these isotopes have the same number of protons. Difference: An S-32 atom has 16 neutrons, an S-33 atom has 17 neutrons, an S-34 atom has 18 neutrons, and an S-36 atom has 20 neutrons. Similarity: Every sulfur atom has 16 protons. Difference: The number of neutrons in an atom of one isotope is different than the number of neutrons in an atom of a different isotope. MY Answer= Sim. # protons Differ. # neutrons (It is the definition of an isomer.) |
64 In the space in your answer booklet, draw a Lewis electron-dot diagram for an atom of sulfur-33. [1]
LINK TO SEE THE ANSWER
65 In the space in your answer booklet, show a correct numerical setup for calculating the atomic mass of sulfur. [1]
HIGHLIGHT TO SEE THE ANSWER
(31.97)(0.9493) + (32.97)(0.0076) + (33.97)(0.0429) + (35.97)(0.0002)or (31.97)(94.93) + (32.97)(0.76) + (33.97)(4.29) + (35.97)(0.02)100 DO NOT SOLVE |
