Questions
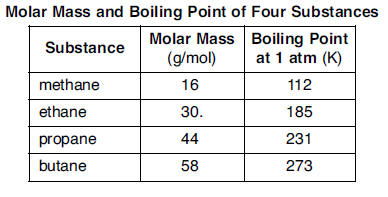 54 On the grid in your answer booklet, mark an appropriate scale on the axis labeled “Boiling Point (K).” [1]
55 On the same grid, plot the data from the data table. Circle and connect the points. [1] LINK TO SEE THE ANSWERS 54 and 55 56 Based on the data in the table, state the relationship between the boiling point at 1 atmosphere and molar mass for these four substances. [1] HIGHLIGHT TO SEE THE ANSWER
57 State, in terms of intermolecular forces, why the boiling point of propane at 1 atmosphere is lower than the boiling point of butane at 1 atmosphere. [1] HIGHLIGHT TO SEE THE ANSWER
|